Vascular Regulation of Hematopoietic Stem Cell Ontogeny
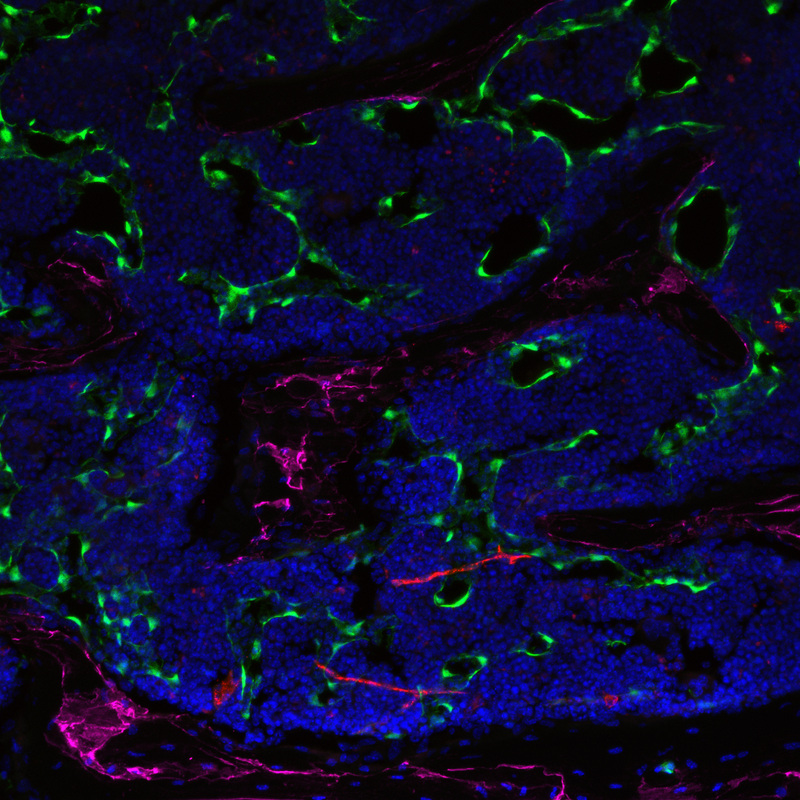
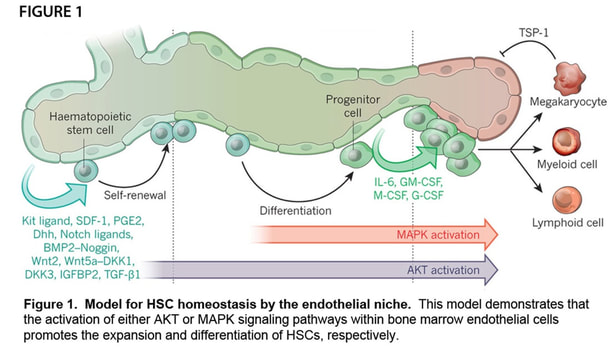
Our laboratory has focused on enhancing regeneration of the hematopoietic system in the context of hematological injuries (e.g. myelosuppression), leukemogenesis, and aging. Our commitment to studying the hematopoietic system is based on the discoveries by our groupdemonstrating that bone marrow endothelial cells (BM ECs) serve as instructive niche cells that are essential for the reconstitution of the hematopoietic system following radiation injury (Figure 1). Our approach proved that the cellular cross talk between the BM vascular niche and hematopoietic stem cells (HSCs) is largely dependent on the activation state of the ECs. These innovative concepts have lead to a paradigm shift in vascular biology, demonstrating that BM ECs are not only essential for the delivery of oxygen, nutrients, and waste disposal, but also function as a specialized vascular niche that, upon proper activation, can instructively support the maintenance and reconstitution of normal HSCs. The concept that ECs provide an instructive fertile niche for maintenance of functional HSCs opens up new avenues for many aspects in stem cell biology and can be applied to multiple biosystems.
Our laboratory is currently aiming to identify relevant paracrine factors and metabolites found within the BM vascular niche cells that modulate self-renewal and expansion of adult HSCs and to develop strategies to enhance hematopoietic reconstitution in the clinical arena. In addition to studying the role of the ECs in regulating adult hematopoiesis, we are interrogating the ability of embryonic vascular niches to induce and expand definitive HSCs from pluripotent stem cell (PSC) sources. In order to determine the optimal embryonic vascular niche for the induction and expansion of embryonic-stage hematopoietic stem/progenitor cells (HSPCs), we have developed methods to isolate and culture ECs from numerous fetal and adult hematopoietic tissues. Furthermore, we have developed an effective, novel platform to expand adult BM macaque CD34+ LT-HSCs and iPSC-HSPCs on human ECs that have high levels of engraftment in NSG mice. This evidence substantiates our novel approach to enhance definitive HSPCs through direct contact cultures with pro-hematopoietic signals unique to ECs. It is our long-term goal to deconstruct the critical stage-specific signal pathways differentially required for induction and expansion of HSCs from embryonic sources as well as enabling the use of patient-specific cells to safely treat their hematological deficiencies.
Overall, our laboratory is dedicated to understanding the role of tissue-specific ECs in establishing unique instructive vascular niche cells that produce the correct milieu and stoichiometry of paracrine factors to direct organ regeneration, in particular within the BM microenvironment. We have assembled large transcriptional profiles from tissue-specific vascular niches that have revealed remarkable heterogeneity within the adult vasculature that is underscored by the production of tissue-specific paracrine factors which we believe are necessary for orchestrating the regeneration of their organ of origin. Based on these data, we are developing novel therapeutic applications and justification for the transplantation of genetically modified BMECs or other cellular sources that are directly reprogrammed into BMECs utilizing a unique set of transcription factors that establish BMEC identity to enhance the regeneration of the BM microenvironment. We hypothesize that reconstitution of hematopoiesis along with an increase in engrafted ECs will not only cooperatively augment both the stability and integrity of the newly formed vessels but also via an inductive mechanism to promote tissue repair and multi-organ regeneration. We expect that the knowledge gained from my laboratory’s research goals will have a significant and positive impact on decreasing the morbidity and mortality associated with life threatening pancytopenia that is associated with hematopoietic dysfunction.
The long-term vision of our research is to demonstrate that ECs can rapidly and efficiently home and functionally engraft multiple organ-specific vascular beds. These findings will allow us to study the role of transplanted ECs in a vast array of biological systems that require the stimulation of organ regeneration. The proposed work is expected to challenge the scientific conceptualization of a monofunctional, relatively inert, microvasculature by revealing a dynamic role for ECs in injury repair and regeneration. We are hopeful that the endless pursuit of our laboratory’s vision and potential fruitful collaborations throughout the scientific community will open up innovative avenues for translational cellular therapy.
Our laboratory has focused on enhancing regeneration of the hematopoietic system in the context of hematological injuries (e.g. myelosuppression), leukemogenesis, and aging. Our commitment to studying the hematopoietic system is based on the discoveries by our groupdemonstrating that bone marrow endothelial cells (BM ECs) serve as instructive niche cells that are essential for the reconstitution of the hematopoietic system following radiation injury (Figure 1). Our approach proved that the cellular cross talk between the BM vascular niche and hematopoietic stem cells (HSCs) is largely dependent on the activation state of the ECs. These innovative concepts have lead to a paradigm shift in vascular biology, demonstrating that BM ECs are not only essential for the delivery of oxygen, nutrients, and waste disposal, but also function as a specialized vascular niche that, upon proper activation, can instructively support the maintenance and reconstitution of normal HSCs. The concept that ECs provide an instructive fertile niche for maintenance of functional HSCs opens up new avenues for many aspects in stem cell biology and can be applied to multiple biosystems.
Our laboratory is currently aiming to identify relevant paracrine factors and metabolites found within the BM vascular niche cells that modulate self-renewal and expansion of adult HSCs and to develop strategies to enhance hematopoietic reconstitution in the clinical arena. In addition to studying the role of the ECs in regulating adult hematopoiesis, we are interrogating the ability of embryonic vascular niches to induce and expand definitive HSCs from pluripotent stem cell (PSC) sources. In order to determine the optimal embryonic vascular niche for the induction and expansion of embryonic-stage hematopoietic stem/progenitor cells (HSPCs), we have developed methods to isolate and culture ECs from numerous fetal and adult hematopoietic tissues. Furthermore, we have developed an effective, novel platform to expand adult BM macaque CD34+ LT-HSCs and iPSC-HSPCs on human ECs that have high levels of engraftment in NSG mice. This evidence substantiates our novel approach to enhance definitive HSPCs through direct contact cultures with pro-hematopoietic signals unique to ECs. It is our long-term goal to deconstruct the critical stage-specific signal pathways differentially required for induction and expansion of HSCs from embryonic sources as well as enabling the use of patient-specific cells to safely treat their hematological deficiencies.
Overall, our laboratory is dedicated to understanding the role of tissue-specific ECs in establishing unique instructive vascular niche cells that produce the correct milieu and stoichiometry of paracrine factors to direct organ regeneration, in particular within the BM microenvironment. We have assembled large transcriptional profiles from tissue-specific vascular niches that have revealed remarkable heterogeneity within the adult vasculature that is underscored by the production of tissue-specific paracrine factors which we believe are necessary for orchestrating the regeneration of their organ of origin. Based on these data, we are developing novel therapeutic applications and justification for the transplantation of genetically modified BMECs or other cellular sources that are directly reprogrammed into BMECs utilizing a unique set of transcription factors that establish BMEC identity to enhance the regeneration of the BM microenvironment. We hypothesize that reconstitution of hematopoiesis along with an increase in engrafted ECs will not only cooperatively augment both the stability and integrity of the newly formed vessels but also via an inductive mechanism to promote tissue repair and multi-organ regeneration. We expect that the knowledge gained from my laboratory’s research goals will have a significant and positive impact on decreasing the morbidity and mortality associated with life threatening pancytopenia that is associated with hematopoietic dysfunction.
The long-term vision of our research is to demonstrate that ECs can rapidly and efficiently home and functionally engraft multiple organ-specific vascular beds. These findings will allow us to study the role of transplanted ECs in a vast array of biological systems that require the stimulation of organ regeneration. The proposed work is expected to challenge the scientific conceptualization of a monofunctional, relatively inert, microvasculature by revealing a dynamic role for ECs in injury repair and regeneration. We are hopeful that the endless pursuit of our laboratory’s vision and potential fruitful collaborations throughout the scientific community will open up innovative avenues for translational cellular therapy.
Program Overview
Adult hematopoietic stem cells (HSCs) are defined by their ability to undergo self-renewal and maintain the capacity to generate all of the mature hematopoietic cell types within the blood and immune system. These unique qualities make the HSC clinically useful in bone marrow (BM) transplantation settings for a wide variety of hematological diseases. Despite advances in the understanding of HSC biology, the exact mechanisms that regulate the balance between self-renewal and lineage-specific differentiation are still unknown. Maintenance of HSCs is dependent upon the cell-intrinsic properties of the HSC, as well as the extrinsic cues of the BM microenvironment. Within the hematopoietic microenvironment, we have found that endothelial cells (ECs) are indispensable in supporting HSC self-renewal and differentiation into lineage-committed progeny to maintain homeostatic hematopoiesis through Akt-mediated signaling (Figure 1). Furthermore, we have demonstrated that Akt-activation endows ECs with the instructive capacity to support the self-renewal of HSCs to maintain homeostasis and promote the regeneration of the hematopoietic system following myeloablative stress through the expression of pro-hematopoietic paracrine factors (herein referred to as angiocrine factors), whereas MAPK/NF-kB signaling promotes HSC differentiation into lineage committed progenitors. The overall goal of our research program is to determine how modulating signaling pathways downstream of Akt within ECs promotes the maintenance, self-renewal and regenerative capacity of the hematopoietic system. By modulating these pathways, we aim to identify novel mechanisms by which the BM vascular niche facilitates the maintenance and expansion of HSCs, improving the cellular fitness and metabolic health of the HSC. Modulating the signaling machinery in ECs may also be used to promote vascular regeneration and to enhance the instructive function of BMECs, offering novel therapeutic approaches to accelerate the regeneration of the HSC pool. Additionally, we will be able to utilize our technologies to expand transplantable, repopulating HSCs ex vivo, thereby ameliorating life-threatening pancytopenia associated with chemo-irradiation. Identification of a more robust ex vivo expansion system will help build upon current HSC expansion strategies and will have a tremendous impact on the therapeutic use of HSCs for the treatment of hematological disorders.
Below is a brief description of our three main projects aimed at defining the role of the vascular niche during the induction of developmental hematopoiesis and regulation of normal and malignant hematopoiesis throughout hematopoietic ontogeny.
Project 1: The role of Akt signaling in regulating functional hematopoiesis.
The only cure for many hematopoietic disorders affecting the BM and/or blood cells is a HSC transplant. The overarching goal of this research project is to identify the signaling pathways within BM ECs that will improve upon ex vivo protocols to expand clinically relevant levels of HSCs for transplantation. Additionally, we aim to identify novel mechanisms by which the BM vascular niche supports the self-renewal and differentiation of the HSC. We have generated preliminary data that demonstrate that EC-specific Akt activation, in combination with NF-kB inhibition, promotes the self-renewal and expansion of the HSC pool in vitro and in vivo and enhances the regeneration of the hematopoietic system following myeloablative insult. Based on this evidence, we hypothesize that inhibition of the NF-kB signaling pathway in ECs results in the increased interaction of HSCs with the vascular niche thereby promoting the self-renewal and regenerative capacity of the HSC. By modulating the NF-kB pathway in BM ECs, we will gain novel insights as to how the BM microenvironment regulates HSC maintenance. Additionally, we have unlocked a novel therapeutic application and justification for the transplantation of genetically modified BM ECs following myeloablative treatment. We have also developed a novel vascular platform that allows for the robust expansion of human umbilical cord blood (UCB) hematopoetic stem and progenitor cells (HSPCs) in a co-culture system. We will test whether inhibiting the NF-kB signaling pathway in our vascular feeder layer will enhance the expansion, homing, and engraftment of expanded human UCB products. Improving upon our ex vivo expansion protocol, along with the therapeutic transplantation of BM ECs, we aim to create a more permissive microenvironment that promotes an increase in the number of engrafted HSCs following BM transplantation, accelerating the rate of hematopoietic recovery following radiation or chemotherapeutic regimens.
Project 2: Determine if aged BM ECs supports and/or initiates aged-related leukemia
The long-term objective of this project is to define the mechanisms by which the aged BM vascular niche leads to the functional decline in aged HSCs, as well as to define the mechanisms by which dysregulation in the aged BM vascular niche leads to the initiation and progression of age-associated acute myeloid leukemia (AML). Aging leads to an increased susceptibility to acquiring life-threatening diseases and infections. Studies have shown that intrinsic alterations in the HSC occur during the aging process, which lead to many defects in the functional properties of the HSC pool, including a decrease in the body’s ability to mount an effective and proper immune response. However, the exact cellular and molecular pathways involved in the physiological aging of HSCs are not fully elucidated. In most cases, the absolute number of HSCs increases with age; however, aged HSCs experience a decrease in their functional potential and self-renewing capabilities. Transplantation studies have demonstrated that age-related changes have a therapeutically relevant impact on engraftment efficiencies and multi-lineage reconstitution, leading to a significant myeloid bias at the expense of lymphopoiesis, suggesting a causative role for the initiation and progression of many hematopoietic malignancies seen in elderly patients. To date, many studies have demonstrated that age-related leukemogenesis is primarily a cell intrinsic defect within the hematopoietic system, but several of these studies have not taken into account the potential effects of the aged microenvironment.
There is significant evidence demonstrating a functional cellular cross talk between the HSC and its niche. We have generated data that indicate that the activation state of aged BM vascular niche interferes with the capacity of aged ECs to support hematopoiesis. Based on this evidence, we hypothesize that disruption of downstream signaling pathways of Akt signaling in aged BM ECs will perturb the interaction of HSCs with the vascular niche, resulting in defects in HSC function that lead to hematopoietic malignancies. Our laboratory is employing in vivo and in vitro vascular niche models to identify alterations in signaling pathways of ECs. These models will determine if the disruption of the intimate cross talk between the BM vascular niche and the hematopoietic system may lead to the improper production of angiocrine factors that result in decreased HSC function and contribute to the onset of age-related hematopoietic malignancies such as AML.
Project 3: Generate definitive hematopoietic stem cells from pluripotent stem cells
Endothelial cells serve an integral role in the supportive niche of HSCs throughout development, from the first site of HSC generation in the dorsal aorta of the embryonic AGM (aorta-gonad-mesonephros) region, to the EC sinusoids of the fetal liver where HSCs undergo significant proliferative expansion, and finally in BM where sinusoidal ECs play a role in adult HSC homeostasis. Therefore, the in vitro recapitulation of critical signal pathways that regulate these developmentally distinct EC hematopoietic niche environments which are involved in establishing, maintaining, and expanding HSCs throughout development will be valuable in the pursuit to generate and expand HSCs from pluripotent stem cells. Our laboratory has begun to use the mouse embryo as a model system to further explore developmental-specific vascular niches to support embryonic-derived HSCs, with our long-term goal to utilize ex vivo niches to expand ESC and iPSC-derived HSPC capable of in vivo engraftment.
Adult hematopoietic stem cells (HSCs) are defined by their ability to undergo self-renewal and maintain the capacity to generate all of the mature hematopoietic cell types within the blood and immune system. These unique qualities make the HSC clinically useful in bone marrow (BM) transplantation settings for a wide variety of hematological diseases. Despite advances in the understanding of HSC biology, the exact mechanisms that regulate the balance between self-renewal and lineage-specific differentiation are still unknown. Maintenance of HSCs is dependent upon the cell-intrinsic properties of the HSC, as well as the extrinsic cues of the BM microenvironment. Within the hematopoietic microenvironment, we have found that endothelial cells (ECs) are indispensable in supporting HSC self-renewal and differentiation into lineage-committed progeny to maintain homeostatic hematopoiesis through Akt-mediated signaling (Figure 1). Furthermore, we have demonstrated that Akt-activation endows ECs with the instructive capacity to support the self-renewal of HSCs to maintain homeostasis and promote the regeneration of the hematopoietic system following myeloablative stress through the expression of pro-hematopoietic paracrine factors (herein referred to as angiocrine factors), whereas MAPK/NF-kB signaling promotes HSC differentiation into lineage committed progenitors. The overall goal of our research program is to determine how modulating signaling pathways downstream of Akt within ECs promotes the maintenance, self-renewal and regenerative capacity of the hematopoietic system. By modulating these pathways, we aim to identify novel mechanisms by which the BM vascular niche facilitates the maintenance and expansion of HSCs, improving the cellular fitness and metabolic health of the HSC. Modulating the signaling machinery in ECs may also be used to promote vascular regeneration and to enhance the instructive function of BMECs, offering novel therapeutic approaches to accelerate the regeneration of the HSC pool. Additionally, we will be able to utilize our technologies to expand transplantable, repopulating HSCs ex vivo, thereby ameliorating life-threatening pancytopenia associated with chemo-irradiation. Identification of a more robust ex vivo expansion system will help build upon current HSC expansion strategies and will have a tremendous impact on the therapeutic use of HSCs for the treatment of hematological disorders.
Below is a brief description of our three main projects aimed at defining the role of the vascular niche during the induction of developmental hematopoiesis and regulation of normal and malignant hematopoiesis throughout hematopoietic ontogeny.
Project 1: The role of Akt signaling in regulating functional hematopoiesis.
The only cure for many hematopoietic disorders affecting the BM and/or blood cells is a HSC transplant. The overarching goal of this research project is to identify the signaling pathways within BM ECs that will improve upon ex vivo protocols to expand clinically relevant levels of HSCs for transplantation. Additionally, we aim to identify novel mechanisms by which the BM vascular niche supports the self-renewal and differentiation of the HSC. We have generated preliminary data that demonstrate that EC-specific Akt activation, in combination with NF-kB inhibition, promotes the self-renewal and expansion of the HSC pool in vitro and in vivo and enhances the regeneration of the hematopoietic system following myeloablative insult. Based on this evidence, we hypothesize that inhibition of the NF-kB signaling pathway in ECs results in the increased interaction of HSCs with the vascular niche thereby promoting the self-renewal and regenerative capacity of the HSC. By modulating the NF-kB pathway in BM ECs, we will gain novel insights as to how the BM microenvironment regulates HSC maintenance. Additionally, we have unlocked a novel therapeutic application and justification for the transplantation of genetically modified BM ECs following myeloablative treatment. We have also developed a novel vascular platform that allows for the robust expansion of human umbilical cord blood (UCB) hematopoetic stem and progenitor cells (HSPCs) in a co-culture system. We will test whether inhibiting the NF-kB signaling pathway in our vascular feeder layer will enhance the expansion, homing, and engraftment of expanded human UCB products. Improving upon our ex vivo expansion protocol, along with the therapeutic transplantation of BM ECs, we aim to create a more permissive microenvironment that promotes an increase in the number of engrafted HSCs following BM transplantation, accelerating the rate of hematopoietic recovery following radiation or chemotherapeutic regimens.
Project 2: Determine if aged BM ECs supports and/or initiates aged-related leukemia
The long-term objective of this project is to define the mechanisms by which the aged BM vascular niche leads to the functional decline in aged HSCs, as well as to define the mechanisms by which dysregulation in the aged BM vascular niche leads to the initiation and progression of age-associated acute myeloid leukemia (AML). Aging leads to an increased susceptibility to acquiring life-threatening diseases and infections. Studies have shown that intrinsic alterations in the HSC occur during the aging process, which lead to many defects in the functional properties of the HSC pool, including a decrease in the body’s ability to mount an effective and proper immune response. However, the exact cellular and molecular pathways involved in the physiological aging of HSCs are not fully elucidated. In most cases, the absolute number of HSCs increases with age; however, aged HSCs experience a decrease in their functional potential and self-renewing capabilities. Transplantation studies have demonstrated that age-related changes have a therapeutically relevant impact on engraftment efficiencies and multi-lineage reconstitution, leading to a significant myeloid bias at the expense of lymphopoiesis, suggesting a causative role for the initiation and progression of many hematopoietic malignancies seen in elderly patients. To date, many studies have demonstrated that age-related leukemogenesis is primarily a cell intrinsic defect within the hematopoietic system, but several of these studies have not taken into account the potential effects of the aged microenvironment.
There is significant evidence demonstrating a functional cellular cross talk between the HSC and its niche. We have generated data that indicate that the activation state of aged BM vascular niche interferes with the capacity of aged ECs to support hematopoiesis. Based on this evidence, we hypothesize that disruption of downstream signaling pathways of Akt signaling in aged BM ECs will perturb the interaction of HSCs with the vascular niche, resulting in defects in HSC function that lead to hematopoietic malignancies. Our laboratory is employing in vivo and in vitro vascular niche models to identify alterations in signaling pathways of ECs. These models will determine if the disruption of the intimate cross talk between the BM vascular niche and the hematopoietic system may lead to the improper production of angiocrine factors that result in decreased HSC function and contribute to the onset of age-related hematopoietic malignancies such as AML.
Project 3: Generate definitive hematopoietic stem cells from pluripotent stem cells
Endothelial cells serve an integral role in the supportive niche of HSCs throughout development, from the first site of HSC generation in the dorsal aorta of the embryonic AGM (aorta-gonad-mesonephros) region, to the EC sinusoids of the fetal liver where HSCs undergo significant proliferative expansion, and finally in BM where sinusoidal ECs play a role in adult HSC homeostasis. Therefore, the in vitro recapitulation of critical signal pathways that regulate these developmentally distinct EC hematopoietic niche environments which are involved in establishing, maintaining, and expanding HSCs throughout development will be valuable in the pursuit to generate and expand HSCs from pluripotent stem cells. Our laboratory has begun to use the mouse embryo as a model system to further explore developmental-specific vascular niches to support embryonic-derived HSCs, with our long-term goal to utilize ex vivo niches to expand ESC and iPSC-derived HSPC capable of in vivo engraftment.